
Recent changes in TGA Medical Device Regulations
In December 2020, TGA released a new Personalised Medical Device Guideline along with a suite of new names and definitions that have shaken up the niche and specialised Custom-made Medical Devices sector.
The new guidance reflects amendments to the Therapeutic Goods (Medical Devices) Regulations 2002, meaning that compliance is mandatory.
Some of the main regulatory changes are –
- Personalised Medical Devices, along with four new sub-categories, now replace the former Custom-made Medical Devices
- The new sub-categories of Personalised Medical Devices are: custom-made, patient-matched, medical device production system (MDPS), and adaptable medical devices
- The majority of the devices currently supplied under the previous exemption will need to be included on the ARTG
- Sponsors and manufacturers must now determine the device’s new definition and take appropriate transition activities
Drivers for change
Technology advancements mean personalised devices are more accessible than ever, but regulations haven’t kept up with the rate of change. Many personalised devices now in the market (such as stent grafts and hip implants) are higher-risk; but escaped the full brunt of TGA compliance measures due to exemptions under the former model.
Similar misalignment is common internationally and so regulators are working towards harmonised reform. Harmonisation and mutual recognition are vitally important to the viability of the Australian sector as our local device market accounts for only 2% of the global market.
Finally, an imbalance between the risk associated with personalised medical devices and regulatory oversight has developed over time. Previously, responsibility for safety, performance and quality of personalised devices sat with the commissioning healthcare professionals. As these devices have become more advanced and high-risk use has increased, it is no longer appropriate for medical practitioners to bear this risk.
What do YOU Need to Do?
1. Check against the guideline to determine the new definition of your device (‘custom-made’, ‘patient-matched’, ‘MDPS’, or ‘adaptable’). Ensuring the correct new definition of your device creates a clear transition pathway.
2. Confirm the transition timelines for your device. You may be required to contact TGA about your device before 01 August 2021!
Download our Transition Timeline Infographic now to help you navigate through the key timelines for each personalised medical device category.
Transition Timelines for Personalised Medical Devices in Australia
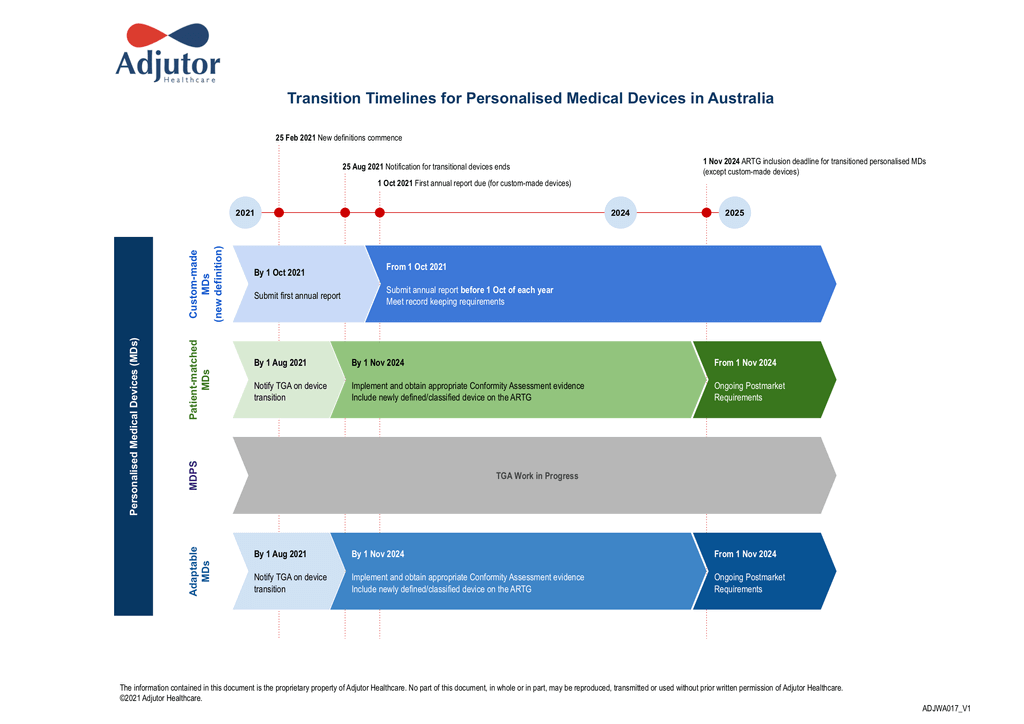
3. Then , you may need to undertake appropriate conformity assessment procedure and include your device on the ARTG by 01 November 2024.
Our Global Specialists Can Help You
